Structural and biochemical characterization of novel tools in optogenetics
 | Prof. Dr. Gebhard F. X. Schertler Paul Scherrer Institut OFLC/109 CH-5232 Villigen PSI Laboratory of Biomolecular Research, PSI +41 56 310 4265 | ||||
| Project starts | 1.1.2016 | ||||
| Project ends | 30.6.2018 | ||||
| Goals | Optogenetics has revolutionized neurobiology by allowing researchers to non-invasively control neuronal activity in live animals. It currently relies primarily on light-sensitive proteins (photopigments) that can be used to modulate ionic conductance of cell membranes and is therefore restricted to cell types which can be stimulated with ion fluxes such as neurons and myocytes. The goal of our study is to expand the reach of optogenetics by developing new tools that would allow for a light- triggered control of any G-Protein Coupled Receptor (GPCR) - dependent cellular signaling pathway. The GPCR protein family is one of the most important families of membrane receptors in all eukaryotes and mostly facilitates optical, chemical and hormone induced signaling. GPCR signaling primarily operates by activating heterotrimeric G proteins (Gαβγ) and arrestins. The cellular response upon receptor activation is mostly dependent on the identity of the Gα protein to which the receptor couples and GPCRs influence a wide array of cellular processes such as vison, muscle contraction, glucose/glycogen concentration and many more. Being able to photoactivate any GPCR signaling pathway will dramatically increase the scope and possibilities of optogenetics, and will allow for a precise spatial and temporal activation of defined signaling pathways in a living organism. In order to reach our goal we identified so-called bistable pigments, mostly from invertebrates, as being the most suitable base for our project, since they can photochemically self-regenerate in any tissue. | ||||
| Contact |
| ||||
| Abstract | We aim to engineer new optogenetic tools by fusing bistable pigments with functional intracellular domains of different GPCRs to obtain control over divers GPCR signaling pathways. Using cutting edge methods in structure determination and advanced computational modeling will enable us to design new receptor variants with modified spectral sensitivity and targeting specific G protein dependent and MAP Kinase signaling pathways. To reach our objective, we need a much better knowledge of the structural properties and dynamics of bistable pigments. Due to the very fast nature of the photoactivation process, bistable pigments crystals will be ideal samples for pump-probe experiments of time-resolved crystallography at X-ray femtosecond (X-FEL) beamlines. For an overview see Neutze et al. “Membrane protein structural biology using X-ray free electron lasers”, Curr Opin Struct Biol. 2015 Aug; 33:115-25. These experiments will be performed at the SwissFEL facility at the Paul Scherrer Institute. A typical experiment would be crystallizing a bistable pigment in a dark state into a viscous solution (i.e. lipidic cubic phase, LCP). Then, a continuous stream of microcrystals is injected across the X-FEL beam by a microjet and X-ray diffraction patterns are recorded. Before injection, the crystal proteins are activated through illumination by a femtosecond laser of the appropriate wavelength with a defined time delay allowing for the snapshot recording of reaction intermediates. Since Retinal isomerization take from femtoseconds to several picoseconds and femtosecond laser pulses are required to allow for a sufficient short time delay to measure/catch this ultrafast process. Using molecular dynamics and molecular modeling we can exploit these results on receptor dynamics to guide the protein engineering. 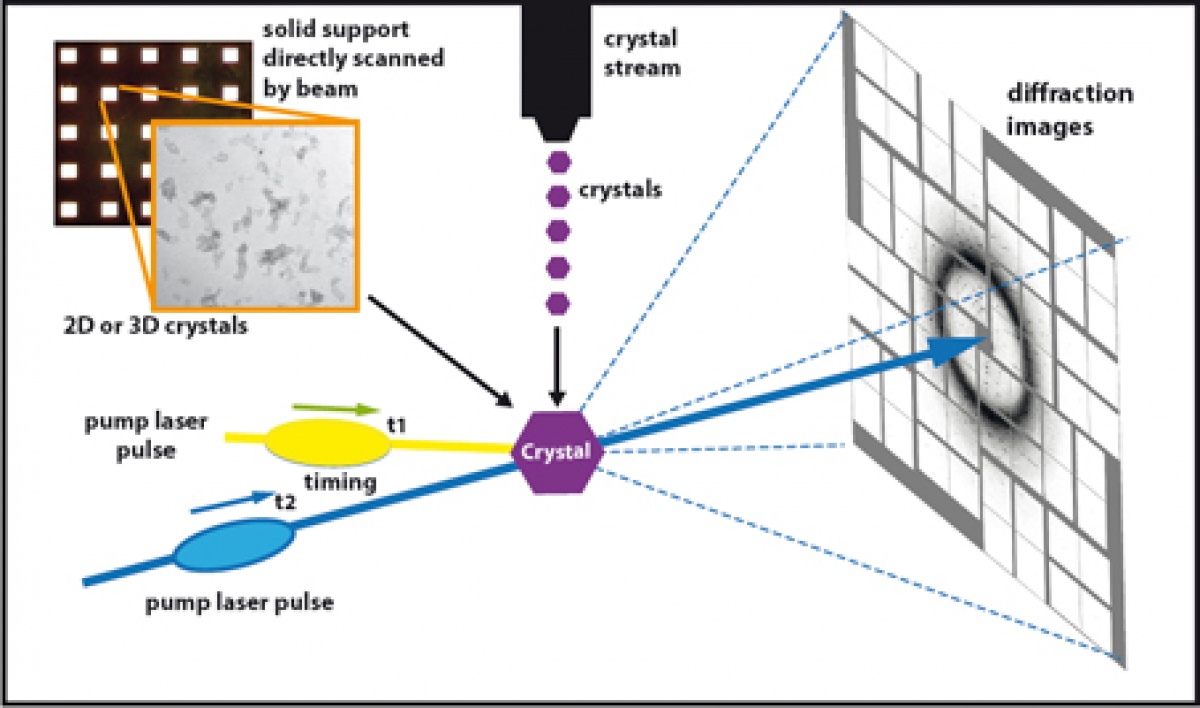 Fig. 1. Schematic drawing of pump–probe time-resolved serial femtosecond crystallography experimental setup for retinal protein dynamics study. The fi gure depicts the overall setup containing the optical pump laser (yellow), the X-ray FEL pulse (blue) and the detectors with an di ffraction pattern (white). The high X-FEL intensities used in the “di ffract before destroy” regime of SFX require a constant delivery of crystals at room temperature, precisely aligned with the path of the pump laser activating the retinal protein at t1. After a defi ned time-delay t2, the X-FEL pulse is delivered resulting in a di ffraction pattern speci c for the conformational arrangement of the protein at t2. Varying t2 results in a series of intermediate “snapshots” of the light-induced conformational change. Currently, two sample delivery modes are available for SFX, a fi xed target wafers with arrays of windows with painted 2D or 3D crystals in a glucose-containing solution, and a microjet system delivering a liquid stream of submicron crystals. Figure adapted from Panneels et al. “Time-resolved structural studies with serial crystallography: A new light on retinal proteins.” Struct Dyn. 2015 Jun 29;2(4):041718. | ||||
| Publications |
|

 Ursula Keller wins “Swiss Nobel” Marcel Benoist Prize
Ursula Keller wins “Swiss Nobel” Marcel Benoist Prize Farewell: the NCCR MUST ended
Farewell: the NCCR MUST ended  MUST2022 Conference
MUST2022 Conference New scientific highlights
New scientific highlights FELs of Europe prize for Jeremy Rouxel
FELs of Europe prize for Jeremy Rouxel Ruth Signorell wins Doron prize
Ruth Signorell wins Doron prize New FAST-Fellow Uwe Thumm at ETH
New FAST-Fellow Uwe Thumm at ETH International Day of Women and Girls in Science
International Day of Women and Girls in Science New scientific highlight
New scientific highlight EU XFEL Young Scientist Award for Camila Bacellar,
EU XFEL Young Scientist Award for Camila Bacellar, Prizes for Giulia Mancini and Rebeca Gomez Castillo
Prizes for Giulia Mancini and Rebeca Gomez Castillo Nobel Prize in Chemistry awarded to RESOLV Member Benjamin List
Nobel Prize in Chemistry awarded to RESOLV Member Benjamin List Hans Jakob Wörner invited to give the „New Horizons Solvay Lectures”
Hans Jakob Wörner invited to give the „New Horizons Solvay Lectures”  Unusual keynote talk at an international scientific conference
Unusual keynote talk at an international scientific conference NCCR MUST at Scientifica 2021
NCCR MUST at Scientifica 2021